Conclusion: The magnitude of Stelara’s advantages (especially in safety/tolerability) explain why it vastly exceeded Wall Street’s sales expectations.
When Stelara (ustekinumab) was launched in 2009 for psoriasis, Wall Street analysts forecasted 2013 revenues of $500 million. However, by 2012, sales of the drug exceeded $1 billion. Our Clinical Innovation analysis shows ustekinumab to have a significant safety/tolerability advantage over TNF-alpha inhibitors such as Humira (adalimumab), which was considered to be the standard of care (SOC) for moderate-to-severe psoriasis when ustekinumab was launched.
The “Drivers of Improvement” graphic below illustrates the comparison:
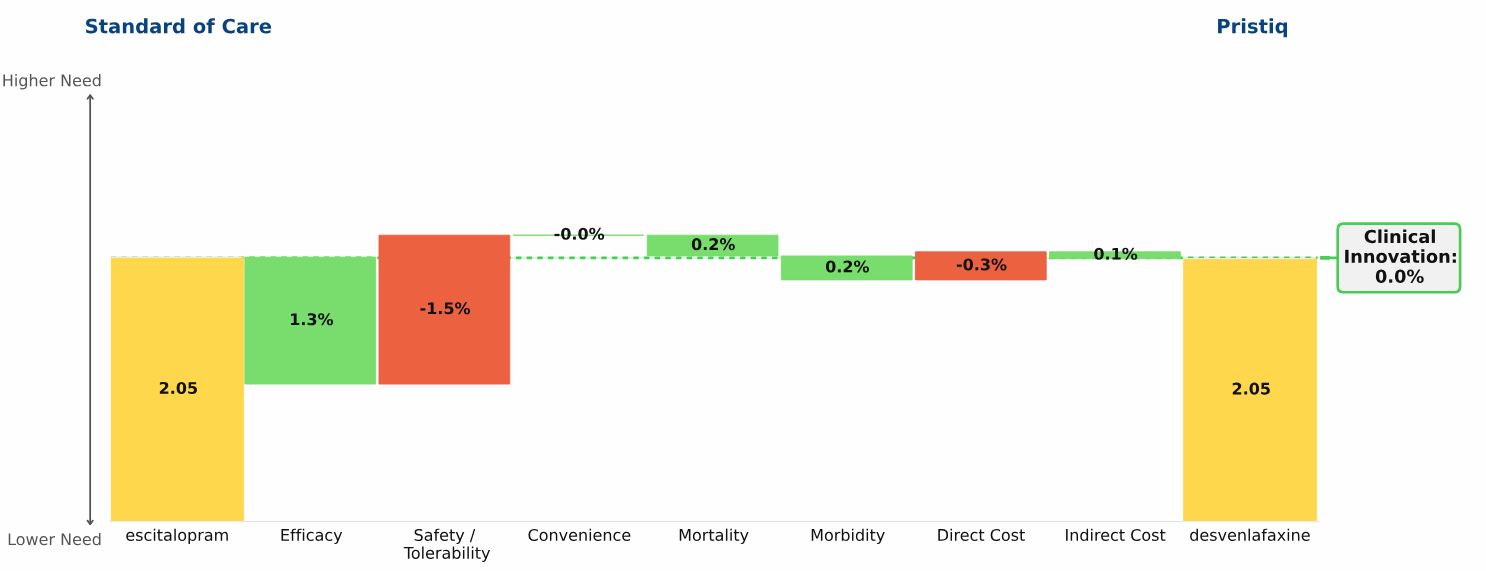
Adalimumab has an unmet need score in moderate-to-severe psoriasis of 1.73, represented by the yellow bar on the left side of the graphic
Ustekinumab’s unmet need score in this population is 1.61, the yellow bar on the right side
Ustekinumab’s Clinical Innovation, or percent reduction in medical need, is 6.6%: Patients treated with ustekinumab have lower unmet need than patients treated with adalimumab
Historically, drugs with 5% or greater Clinical Innovation typically achieve strong patient share.
This graphic also illustrates ustekinumab’s advantages and disadvantages compared with the SOC:
The two drugs have comparable efficacy, with ustekinumab showing a slight advantage over adalimumab
Based on currently available data, ustekinumab has an improved safety/tolerability profile over adalimumab, with no black box warning and lower rates of infection
Ustekinumab is administered as an SC injection once every three months, compared to adalimumab’s twice weekly injections; however, the requirement that ustekinumab be administered in the outpatient setting offsets its frequency advantage
Ustekinumab’s annual drug cost is slightly higher than adalimumab’s
Our analysis shows that ustekinumab offers significant Clinical Innovation over adalimumab, and its commercial performance to date supports this conclusion.
Outlook for IL-17 receptor antagonists
A new class of drugs may soon enter the competitive landscape in moderate-to-severe psoriasis. Amgen, Eli Lilly, and Novartis all have IL-17 receptor antagonists in phaseIIItrials for this indication. Amgen’s product brodalumab has shown the strongest phase II data among the three. Based on these data and assuming price equivalence with ustekinumab, our analysis shows brodalumab to offer a strong 7.3% Clinical Innovation.
While brodalumab and ustekinumab show a similar percentage of patients responding (90% and 91%, respectively achieving at least a 50% PASI improvement at 12 weeks), almost twice as may brodalumab patients as ustekinumab patients (74% vs. 38%) reach at least 90% PASI improvement. Also, significantly more brodalumab patients have a physician’s global assessment (PGA) of disease as “cleared” or “minimal” (83% vs. 65%). Absent long-term data, we conservatively assume that the percentage of patients maintaining cleared or minimal PGA response at two years will be the same as that seen with ustekinumab.
Our analysis shows brodalumab to have a slight safety/tolerability disadvantage compared with ustekinumab; we assume it will have a black box warning due to a rate of severe bacterial infections comparable to adalimumab’s.
Brodalumab dosing is by SC injection every other week, which our framework considers slightly less convenient than an outpatient injection once every three months.
In our view, the Clinical Innovation of brodalumab over ustekinumab is similar to that shown by ustekinumab over adalimumab. If phase III trials of brodalumab replicate the efficacy and safety/tolerability data seen in phase II, we predict that brodalumab will achieve substantial patient share in the moderate-to-severe psoriasis market.